Welcome to the GCTLC Library. Use the search and filter options below to find green chemistry education resources and curriculum materials from community members from across the world. You can also submit a new resource to the library. For information for authors and reviewers, please consult the Guidelines for Submission and Review of Learning Objects.

Organic Nomenclature and Safety of Chemical Products
This is a discussion prompt where students find a molecule they use in a personal care product, cleaning product, or processed food. They find the structure, relate components of the name to aspects of the structure, look up the product on the Environmental Working Group (EWG) or FDA websites, and look up the molecule in PubChem. At the end, they need to decide whether they are happy with their ...
Organic-Solvent-Free Phase-Transfer Oxidation of Alcohols Using Hydrogen Peroxide
An organic chemistry laboratory experiment illustrating the oxidation of primary and secondary alcohols to their corresponding aldehyde or ketones, respectively, is described. The procedure uses 30% aqueous hydrogen peroxide in the presence of a tungsten catalyst (sodium tungstate) using phase transfer catalysis. The described experiments illustrate basic organic reaction chemistry and techniques ...

Organometallic Nickel(II) Phosphine Complexes for Suzuki-Miyaura Cross-Coupling; A Greener Alternative?
In this experiment, students perform a Grignard reaction under air-free conditions to synthesize and isolate organometallic Ni(II) phosphine complex(es) that are used as pre-catalysts for a Suzuki-Miyaura Coupling reaction in tert-amyl alcohol.
Oxidation of Aromatic Aldehydes Using Oxone
One of the main advantages of doing green chemistry is that it can often be done on the benchtop without the need for a fume-hood. In this experiment water and ethanol are the only solvents used for both the reaction and purification steps and oxone is a safe oxidant whose only by-product is potassium sulfate.
The oxidation of aromatic aldehydes using oxone allows instructors to discuss green ...
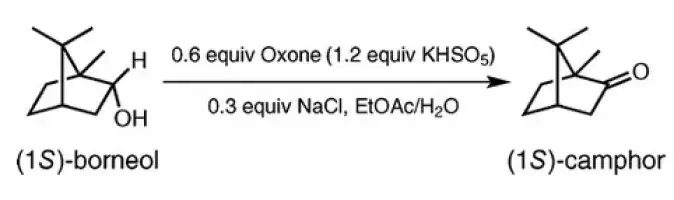
Oxidation of Borneol to Camphor Using Oxone and Catalytic Sodium Chloride: A Green Experiment for the Undergraduate Organic Chemistry Laboratory
A new green oxidation procedure was developed for the undergraduate organic teaching laboratories using Oxone and a catalytic quantity of sodium chloride for the conversion of borneol to camphor. This simple 1 h, room temperature reaction afforded high quality and yield of product, was environmentally friendly, and produced negligible quantities of hazardous waste. The experiment was performed ...
Patterning Self-Assembled Monolayers on Gold. Green Materials Chemistry in the Teaching Laboratory
Applications of organic chemistry to modify the structure and surface properties of materials are becoming increasingly important and interdisciplinary as the dimensions of modern materials decrease. This laboratory exercise illustrates how macroscopic material properties can be modified with self-assembled monolayers and organic thin-film patterning. Using an inexpensive gold on vinyl substrate ...

Pericyclic Reactions
This module teaches fundamentals of pericyclic reactions using examples of biocatalysis, biomimetic catalysis, and natural product total synthesis. After completion, students should be able to identify key disconnections of pericyclic reactions (specifically [4+2] and [2+2] cycloadditions, as well as [3,3]-sigmatropic rearrangements) for the preparation of agrochemicals and natural products (SDG 2 ...

Pilot Project: Chemical Waste Minimization in the Educational Laboratory: Final Report
During Calendar Year 1999, the Illinois Waste Management and Research Center (WMRC), a state agency, and Argonne National Laboratory-East (Argonne), a federal research facility, jointly conducted a pilot project entitled "Chemical Waste Minimization in the Educational Laboratory." Four Chicago area secondary schools voluntarily participated in this Pilot Project. The purpose of the Pilot Project ...

Plastics and Sustainable Building - E-learning course
This self-paced E-learning course aims to show the potentials for sustainable chemistry in the construction sector and to answer the following questions:
> How can plastics be used in a sustainable way in buildings?
> What obstacles are there and how can they be overcome?
> Which steps need to be followed, under consideration of regional differences?
> What are recommendations for ...

Plate to Planet - Lesson 1
*This is lesson 1 of a 4-lesson unit. Green Chemistry technologies are serving as tools to capture the imagination of the next generation of problem solvers. This is a unit investigating biodiversity, food as science, and chemistry as a tool for solving sustainability challenges. The Plate to Planet Unit teaches students in grades 3-5 how food production and food decisions impact our environment ...

Plate to Planet - Lesson 2
*This is lesson 2 of a 4-lesson unit. Green Chemistry technologies are serving as tools to capture the imagination of the next generation of problem solvers. This is a unit investigating biodiversity, food as science, and chemistry as a tool for solving sustainability challenges. The Plate to Planet Unit teaches students in grades 3-5 how food production and food decisions impact our environment ...

Plate to Planet - Lesson 3
*This is lesson 3 of a 4-lesson unit. Green Chemistry technologies are serving as tools to capture the imagination of the next generation of problem solvers. This is a unit investigating biodiversity, food as science, and chemistry as a tool for solving sustainability challenges. The Plate to Planet Unit teaches students in grades 3-5 how food production and food decisions impact our environment ...

Plate to Planet - Lesson 4
*This is lesson 4 of a 4-lesson unit. Green Chemistry technologies are serving as tools to capture the imagination of the next generation of problem solvers. This is a unit investigating biodiversity, food as science, and chemistry as a tool for solving sustainability challenges. The Plate to Planet Unit teaches students in grades 3-5 how food production and food decisions impact our environment ...

Plate to Planet Extension - Elephant Toothpaste
* This is an extension activity to the Plate to Planet Unit. This is an experiment/demonstration that is great for teaching about catalysts. The demonstration will show a chemical reaction in which a catalyst (yeast) is added to hydrogen peroxide to create a foam that looks like a giant squirt of toothpaste that could be used on an elephant!

Plate to Planet Extension - Strawberry DNA Extraction
*This is an extension activity to the Plate to Planet Unit. In this activity, the children will be extracting Deoxyribonucleic Acid, DNA, from strawberries. Cells are the basic unit of life and make up all living things. DNA is the molecule that controls everything in the cell. DNA contains the instructions that direct all the cell’s activities. This lesson will show how DNA can be isolated from ...

Political Engagement in Organic Chemistry
This is a political engagement activity designed for a 2nd year organic chemistry II lecture course. The activity engages the students in the review and summary of pending state legislation. After a class discussion about the content and goals of the bill, the class generates a list of questions they have about the bill. The students are then assigned/select questions to research and collect ...

Pollution in your Home
This feature article, available through the Royal Society of Chemistry (RSC), gives an overview of indoor air pollutants and how chemists and scientists monitor and track them. The article highlights a massive study in June of 2018 that took place in the United States and provides a series of different activities for high school-level students to understand mass spectrometry. It also includes ...

Polymer Chemistry
This module is part of a collection of nine green chemistry teaching modules developed in the early 2000s by a team of faculty (Donna Narsavage-Heald, Trudy Dickneider, David Marx, Timothy Foley, Joan Wasilewski) led by Michael Cann at the University of Scranton and has been migrated to the GCTLC. The subjects of the modules are based on winners of the Green Chemistry Challenge Awards. The modules ...

Polymers and Green Chemistry
The purpose of this lab is to introduce students to some basics of the chemistry of polymers. Polymer chemistry is often an afterthought in the General Chemistry curriculum, but polymers are very much present in students’ lives. The lab introduces the relationship between molecular structure (e.g., branched vs. linear, bulky vs small side groups) and bulk properties (e.g., flexibility). This lab ...

Polymers from Soybeans: The transesterification of triglycerides
This case study explores the transesterification of triglycerides from soybean oil into a variety of vinyl monomers - soy-based acrylic monomers (SBAs). This transesterification uses commercially available N-(hydroxyethyl)acrylamide as a reactant, was carried out in one synthetic step, ran at room temperature (minimizing energy use), and used only a catalytic amount of sodium hydroxide. These ...
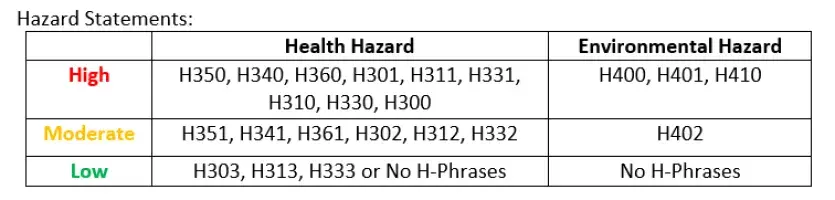
Pre-Lab- Understanding Chemical Hazard and Laboratory- Formula of a Hydrate
In this laboratory experiment, students will perform a hazard assessment for different hydrates and rank them according to their human and environmental hazards. Students will also determine the percent water in an unknown hydrate and use that information to determine the formula of a hydrate.

Preparation and Properties of Lubricant Basestocks from Epoxidized Soybean Oil and 2-Ethylhexanol
This learning object explores the preparation and characterization of lubricant basestocks synthesized from epoxidized soybean oil (ESO) and 2-ethylhexanol (2-EH). The study investigates the reaction pathways—primarily epoxy ring-opening and transesterification—using different catalysts, and evaluates the resulting products in terms of viscosity, pour point, viscosity index, and oxidative ...

Preparation and Testing of Buffers
The purpose of this lab is to give students experience in the practical preparation of buffers and exploring buffer properties. Students will prepare aqueous solutions with specific concentrations of acids and bases, and use pH meters to examine the pH of these buffers. The buffer capacity of the prepared buffers will also be measured.
Featuring contributions from Tamara Fitzjarrald.

Pressure-Sensitive Adhesives Based on Epoxidized Soybean Oil and Dicarboxylic Acids
This learning object examines the development of environmentally friendly pressure-sensitive adhesives (PSAs) synthesized from epoxidized soybean oil (ESO) and bio-based dicarboxylic acids via an epoxide–acid reaction. The study investigates the influence of crosslinking density, monomer ratios, and curing conditions on adhesive performance, including tack, peel strength, and shear resistance. The ...

Primer for the Guide - Green Chemistry: Principles and Lab Practices
This resource has been developed through a collaboration of Beyond Benign, MilliporeSigma, My Green Lab, and a network of chemistry faculty. Green Chemistry: Principles and Lab Practices is an introductory primer for the Guide to Green Chemistry Experiments for Undergraduate Organic Chemistry Labs*, and contains a variety of resources, tools, and references for practicing green chemistry in ...

Principle 1 - Prevent Waste
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 10: Design for Degradation
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 11: Real-Time Analysis for Pollution Prevention
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 12: Inherently Safer Chemistry for Accident Prevention
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 2: Maximize atom economy
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 3: Design less hazardous chemical syntheses
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 4: Design Safer Chemicals and Products
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 5: Safer Solvents and Auxiliaries
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 6: Design for Energy Efficiency
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 7: Use of Renewable Feedstocks
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 8: Reduce Derivatives
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Principle 9: Catalysis
Learn the twelve principles of green chemistry taught during The Green Chemistry & Engineering Student Workshop on June 17, 2013 in Washington D.C. The course content was provided by ACS-GCI. These videos were produced by the Western States Pollution Prevention Network. Other sponsors included NPPR and Washington State Department of Ecology.

Problem Solve Your School-Using engineering design process
This activity is focused on the Engineering Design Process. In the activity, students apply what they have learned about the engineering design process to a real-life problem that affects them and/or their school. They choose a problem as a group, and then follow the engineering design process to come up with and test their design solution. This activity teaches students how to use the engineering ...

Problem-Based Learning in Sustainable Chemistry: A Student Startup Model with PET as a Case Study.
This paper presents a problem-based learning (PBL) framework designed to enhance sustainable chemistry education through a simulated student startup model. Implemented in the MSc Sustainable Chemistry programme at UCL, the activity uses polyethylene terephthalate (PET) as a case study to explore circular economy principles and green chemistry strategies. Students adopt professional roles within ...

Promoting political and civic engagement in a nonmajor sustainable chemistry course
A non-majors chemistry course with a sustainability focus was developed as part of an effort to embed sustainability throughout the curriculum at Widener University. Using the ACS textbook Chemistry in Context, we sought to reinforce the concept of chemistry as the “central science” in solving big issues relating to energy and the environment. What distinguishes this course from traditional ...

Properties of Adhesives: A Sticky Situation: 01 What Do We Learn From Animals?
In this introductory lesson of the Properties of Adhesives: A Sticky Situation unit, students are introduced to biomimicry as a strategy scientists and engineers use to design new technologies. Through word analysis and an interactive matching game, students explore how animals and plants inspire human innovations, including safer adhesives, building designs, and medical materials. The lesson ...

Properties of Adhesives: A Sticky Situation: 02 Testing Tape
In this hands-on laboratory lesson, students investigate the properties of adhesives by testing different types of tape using green chemistry decision-making criteria: performance, safety, and cost. Students measure the force required to remove tape using spring scales, analyze data across multiple trials, and compare results with environmental impact and material sourcing information. By ...

Properties of Adhesives: A Sticky Situation: 03 Battle of the Glues
In this hands-on laboratory lesson, students create and compare two different glues made from household materials to explore how chemical reactions can produce new substances with distinct properties. Students evaluate each glue using green chemistry criteria—cost, safety, and performance—and investigate how starting materials, energy use, and waste generation influence sustainability. Through ...

Properties of Adhesives: A Sticky Situation: 04 Just Glue It!
In this culminating lesson, students synthesize their learning from the A Sticky Situation unit by creating and presenting an advertisement for their homemade glue developed in Lesson 3. Working collaboratively, students use informational texts to analyze real-world adhesive innovations and then communicate why their glue meets green chemistry criteria—cost, safety, and performance. Through ...

Protein N‐Glycans: Incorporating Glycochemistry into the Undergraduate Laboratory Curriculum
The article "Incorporating Glycochemistry into the Undergraduate Laboratory Curriculum: Isolation and Analysis of Soybean Glycoprotein β-Conglycinin" addresses the underrepresentation of glycoscience in undergraduate biochemistry labs by introducing a multiweek experimental series. Students isolate β-conglycinin from soy flour and analyze it using SDS-PAGE and mass spectrometry. They also apply a ...

Putting the squeeze on imine synthesis: citrus juice as a reaction medium in the introductory organic laboratory
This article highlights a less hazardous and energy-efficient organic synthesis utilizing freshly squeezed citrus juice as a solvent that was developed for a sophomore-level laboratory course. The experiment enables students to engage with key green chemistry principles, including waste prevention, atom economy, the use of safer chemicals, and energy efficiency. In the experiment, 4 ...

Qualitative & Quantitative Gas Stoichiometry and Determination of an Unknown Alkali Carbonate
This experiment looks at two types of chemical reactions that produce hydrogen and carbon dioxide gases. It explores both qualitative observations (no measurements or numbers involved) about the reactions, as well as quantitative measurements of the volume of gas produced to explore concepts of stoichiometry and the ideal gas law. These concepts will be used to relate the stoichiometry of gas ...

QUÍMICA VERDE PARA EDUCAÇÃO BÁSICA PARA UMA FORMAÇÃO MAIS SUSTENTÁVEL
This two volume set of "Green Chemistry for Basic Education" analyzes the chemical greenness of classic experimental protocols used in Basic Education (volume I) and then proposes a set of high school level experiments that have greener perspectives and less risk to health and the environment.
Volume I presents green chemistry theory and principles and the Green Matrix used to evaluate classic ...