Scalable manufacturing and reprocessing of vitrimerized flexible polyurethane foam (PUF) based on commercial soy polyols
Summary
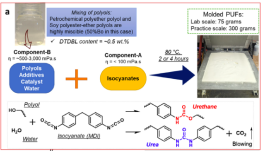
This Learning Object introduces students to Green Chemistry principles through a case study on recyclable flexible polyurethane foams (PUFs) using renewable soy-based polyols. Based on “Scalable manufacturing and reprocessing of vitrimerized flexible polyurethane foam (PUF) based on commercial soy polyols” the study demonstrates how commercial soy polyols can partially replace petrochemical polyols to create recyclable, reprocessable PUFs via vitrimer chemistry.
Students will explore renewable feedstocks, catalysis, material property testing, and dynamic covalent networks for recycling. The resource emphasizes bio-based materials, waste reduction, and product circularity.
Authors & Citation
Authors: Wangcheng Liu, Yaqiong Zhang, Peter Chen, Lin Shao, Yiding Cao, Baoming Zhao, Ellen C. Lee, Xiaojiang Wang, and Jinwen Zhang*
Citation: Liu, W., et al. (2025). Industrial Chemistry & Materials. DOI: 10.1039/d4im00117f
Teaching Use
Ideal for teaching renewable materials, catalysis, polymer recycling, and Green Chemistry life cycle thinking.
Students will explore renewable feedstocks, catalysis, material property testing, and dynamic covalent networks for recycling. The resource emphasizes bio-based materials, waste reduction, and product circularity.
Authors & Citation
Authors: Wangcheng Liu, Yaqiong Zhang, Peter Chen, Lin Shao, Yiding Cao, Baoming Zhao, Ellen C. Lee, Xiaojiang Wang, and Jinwen Zhang*
Citation: Liu, W., et al. (2025). Industrial Chemistry & Materials. DOI: 10.1039/d4im00117f
Teaching Use
Ideal for teaching renewable materials, catalysis, polymer recycling, and Green Chemistry life cycle thinking.
Keyword Tags
