Rheological Properties of Soybean Oil with Nano Additives: A Comprehensive Analysis
Summary
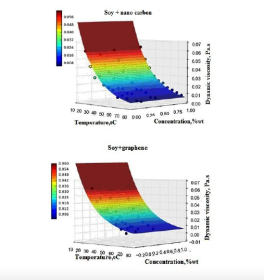
This Learning Object introduces students to the rheological behavior of soybean oil with nano-additives (graphite, graphene, and nanocarbon) as a pathway to developing biodegradable lubricants. Using experimental data and mathematical modeling (power law and temperature-dependent viscosity equations), the study examines how nano-additives influence viscosity, thixotropy, and flow properties. This provides a concrete case study in Green Chemistry, illustrating how renewable, non-toxic, and biodegradable feedstocks such as soybean oil can replace petroleum-based lubricants in industrial and mechanical applications.
For Green Chemistry education, this work highlights key principles: the use of renewable feedstocks, designing safer chemicals and products, and advancing sustainability in materials design. Students will analyze how nano-additive modification can tune material performance, evaluate the environmental benefits compared to synthetic lubricants, and reflect on the role of chemistry in achieving sustainability and the U.N. Sustainable Development Goals.
Relevance to Green Chemistry Learning:
This article is a valuable teaching resource because it connects fundamental physical chemistry concepts (rheology, viscosity, shear stress/strain) with applied sustainability solutions. By investigating soybean oil-based lubricants enhanced with nano-additives, students explore how renewable resources can replace petroleum-derived lubricants while maintaining high performance. This work reinforces the 12 Principles of Green Chemistry, particularly renewable feedstocks, safer design, and designing for degradation, while also connecting chemistry to broader sustainability challenges such as responsible consumption (SDG 12) and climate action (SDG 13).
Citation of Original Work: Stanciu; Asian J. Adv. Res., vol. 7, no. 1, pp. 408-412, 2024.
For Green Chemistry education, this work highlights key principles: the use of renewable feedstocks, designing safer chemicals and products, and advancing sustainability in materials design. Students will analyze how nano-additive modification can tune material performance, evaluate the environmental benefits compared to synthetic lubricants, and reflect on the role of chemistry in achieving sustainability and the U.N. Sustainable Development Goals.
Relevance to Green Chemistry Learning:
This article is a valuable teaching resource because it connects fundamental physical chemistry concepts (rheology, viscosity, shear stress/strain) with applied sustainability solutions. By investigating soybean oil-based lubricants enhanced with nano-additives, students explore how renewable resources can replace petroleum-derived lubricants while maintaining high performance. This work reinforces the 12 Principles of Green Chemistry, particularly renewable feedstocks, safer design, and designing for degradation, while also connecting chemistry to broader sustainability challenges such as responsible consumption (SDG 12) and climate action (SDG 13).
Citation of Original Work: Stanciu; Asian J. Adv. Res., vol. 7, no. 1, pp. 408-412, 2024.
File (PDF, PPT, image, etc)
Link to external