A highly reactive soybean oil-based superhydrophobic polyurethane film with longlasting antifouling and abrasion resistance
Summary
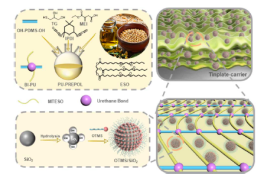
This Learning Object explores the development of a soybean oil-based superhydrophobic polyurethane film that demonstrates long-lasting antifouling and abrasion resistance. The study highlights the synthesis of bio-based polyols derived from epoxidized soybean oil and their subsequent integration into polyurethane coatings enhanced with superhydrophobically modified silica nanoparticles and OH–PDMS–OH. The resulting film maintains its superhydrophobicity (water contact angle >150°) and antifouling performance even after extended exposure to acidic/alkaline environments, outdoor conditions, and mechanical abrasion.
For students in Green Chemistry and Materials Science, this research provides a case study in sustainable polymer design, showing how renewable vegetable oils can substitute for petroleum-derived polyols while delivering robust material performance. It emphasizes both sustainability (biobased raw materials, reduced reliance on fluorinated compounds) and functionality (durability, resistance to fouling and degradation), making it a key example of applying green chemistry principles to industrially relevant materials. This article is important for university-level Green Chemistry education because it illustrates how renewable feedstocks (soybean oil) can be transformed into high-performance materials traditionally made from petroleum sources. It also demonstrates strategies to avoid environmentally persistent fluorinated reagents by using silica nanoparticles and PDMS modifiers. Students can learn how life-cycle considerations—feedstock selection, end-of-life fate, and environmental safety—are integrated into materials innovation. This case study reinforces the 12 Principles of Green Chemistry, particularly renewable feedstocks, design for degradation, and safer materials development.
Citation of Original Work: Nanoscale Adv., 2024,6, 5663-5670. https://doi.org/10.1039/D4NA00674G
For students in Green Chemistry and Materials Science, this research provides a case study in sustainable polymer design, showing how renewable vegetable oils can substitute for petroleum-derived polyols while delivering robust material performance. It emphasizes both sustainability (biobased raw materials, reduced reliance on fluorinated compounds) and functionality (durability, resistance to fouling and degradation), making it a key example of applying green chemistry principles to industrially relevant materials. This article is important for university-level Green Chemistry education because it illustrates how renewable feedstocks (soybean oil) can be transformed into high-performance materials traditionally made from petroleum sources. It also demonstrates strategies to avoid environmentally persistent fluorinated reagents by using silica nanoparticles and PDMS modifiers. Students can learn how life-cycle considerations—feedstock selection, end-of-life fate, and environmental safety—are integrated into materials innovation. This case study reinforces the 12 Principles of Green Chemistry, particularly renewable feedstocks, design for degradation, and safer materials development.
Citation of Original Work: Nanoscale Adv., 2024,6, 5663-5670. https://doi.org/10.1039/D4NA00674G
Link to external