Greener bromination of alkenes using sodium bromide and sodium perborate
Summary
Co-author: Alyssa Gauna
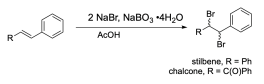
This experiment uses sodium bromide and sodium perborate to generate bromine for the electrophilic bromination of either stilbene or chalcone. Students will then use melting point to determine the stereochemistry of their reaction. They will also use green chemistry metrics to compare the greenness of the procedure.
This experiment uses sodium bromide and sodium perborate to generate bromine for the electrophilic bromination of either stilbene or chalcone. Students will then use melting point to determine the stereochemistry of their reaction. They will also use green chemistry metrics to compare the greenness of the procedure.
File (PDF, PPT, image, etc)
Digital Object Identifier (DOI)
https://doi.org/10.59877/EKBU3163
Creative Commons License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
